In Endo Pharmaceuticals, Inc. v. Actavis, Inc., the Federal Circuit reversed the district court's decision denying Endo's motion for summary judgment of infringement based on an implied license. The Federal Circuit found that Roxane and Actavis did not have an express or implied license to practice the Endo Opana patents at issue, even though they were related to licensed patents.
The Previous ANDA Litigation and License Agreements
The patents at issue relate to Endo's Opana® ER product, which is an extended release formulation of oxymorphone. The parties were involved in earlier ANDA litigation over different Orange Book listed patents for Opana® ER, which was settled by a license and covenant not to sue.
Judge Moore's majority decision for the Federal Circuit discusses the Roxane agreement as representative. As summarized in the decision, the Roxane agreement defines "Licensed Patents" as follows:
- any [U.S.] patents that are both (i) now owned by Endo . . . and (ii) issued as of the Effective Date of this Agreement, including the Opana® ER Patents,
- any [U.S.] patent applications that claim priority to the Opana® ER Patents, including any continuation, continuation-in-part and divisional patent applications that claim priority to Opana® ER Patents, and
- any patents resulting from the reissue or reexamination of patents or patent application of patents or patent applications comprised within clauses (a) and (b) . . .
The "Opana® ER Patents" are defined as U.S. Patents 5,662,933, 5,958,456, and 7,276,250.
The covenant not to sue pertained to "the Licensed Patents" and included a license under "the Licensed Patents." The agreement also included a "No Implied Rights" clause "stating that Endo does not grant to Roxane any license or right 'whether by implication, estoppel or otherwise, other than as expressly granted herein.'"
The Patents at Issue
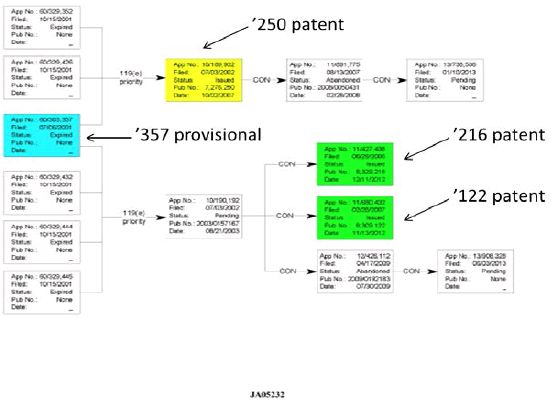
The patents at issue were granted after the settlement and license agreement. U.S. Patent 8,309,122 and U.S. Patent 8,329,216 claim priority to the same provisional application as one of the licensed patents, while U.S. Patent 7,851,482 is not related to the other patents.
The Federal Circuit decision includes this chart showing the relationship of the '122 and '216 patents (green) to the '250 patent (yellow), with the common priority application highlighted in blue.
The District Court Proceedings
Endo asserted the three new patents in a new infringement action, and the defendants opposed on the theories of express and implied license. The district court sympathized with the defendants, and held that "Endo is estopped from claiming that the activity of Actavis and Roxane, which has gone on for a substantial period of time, is now suddenly barred because of these new patents."
The Federal Circuit Decision
The Federal Circuit decision was authored by Judge Moore and joined by Judge Newman. Judge Dyk dissented in part.
The Federal Circuit rejected the defendants' arguments that the new patents were encompassed by the license based on their common priority application.
Roxane's express license arguments are meritless. .... There can be no dispute that the '122 and '216 patents are not continuations of any of the licensed patents .... Likewise, there is no reasonable argument that the '122 and '216 patents claim priority to any of the licensed patents.
The Federal Circuit also rejected the defendants' implied license arguments. The court explained that the TransCore case they relied upon has "a limited scope," and that cases that followed TransCore had involved continuation applications.
Taken together these cases stand for the rule that a license or a covenant not to sue enumerating specific patents may legally estop the patentee from asserting continuations of the licensed patents in the absence of mutual intent to the contrary.
But the Federal Circuit refused to expand the implied license doctrine:
You get what you bargain for. And we will not use the implied license doctrine to insert ourselves into that bargain and rewrite the contract.
Rather than believing that Endo was trying to "tak[e] back in any extent that for which he has already received consideration," the Federal Circuit characterized the defendants as "seek[ing] to capture via implied license subject matter in addition to that for which they bargained."
Endo has granted to Appellees a license and covenant not to sue limited to specific patents and patent applications. If Appellees wanted to market and sell their accused generic products free from any threat of being sued by Endo for patent infringement, they could have negotiated for the appropriate language in the settlement and license agreements. .... Having agreed to licenses that do not cover the patents at issue in these appeals, Appellees will not now be heard to complain.
The court therefore vacated the district court's denials of a preliminary injunction and remanded for further proceedings.
Judge Dyk's Dissent
Judge Dyk joined the majority decision with regard to Roxane, and also "agree[d] that Actavis does not have an implied license to the '482 patent," because Endo did not own that patent at the time of the Actavis settlement agreement. However, Judge Dyk would have found that Actavis had an implied license to the '122 and '216 patents, based on "material differences between the Actavis and Roxane agreements and negotiations."
Part of Judge Dyk's analysis fails to appreciate the difference between an application that claims priority to a provisional application, and a claim that is entitled to the benefit of the filing date of a provisional application. Judge Dyk assumes that "a patent claiming priority to a provisional application must cover the same inventive subject matter as the provisional application," but that is not necessarily true.
Perhaps Judge Dyk lost sight of the fact that, unlike a continuation application, the disclosure of non-provisional application can go beyond that of the provisional application. Moreover, a valid priority claim can be made as long as one claim presented in the later application is described in the earlier application. The decision to make a priority claim usually is made at the time of filing, and only can be changed within a short time period thereafter. Thus, it is not unusual for only some–or even not any–claims of a granted patent to be actually entitled to the benefit of the filing date of its priority provisional application. That is, a patent claiming priority to a provisional application often may cover more than the "inventive subject matter" of the provisional application."
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

