Publicly Traded Life Sciences Companies Remain Prime Targets of Securities Fraud Class Action Lawsuits in the United States
The past year was another noteworthy one with respect to securities fraud class action lawsuits pursued against publicly traded pharmaceutical, biotechnology and medical device companies. In 2011, 17 different life sciences companies (along with their directors, officers, and key personnel) were sued for alleged securities fraud — representing a meaningful decline from the 29 such lawsuits filed in 2010. In terms of substance, the 2011 securities fraud lawsuits saw a relative return to a focus on industry-specific issues as compared to claims of financial improprieties, with allegations of improper marketing practices being the most prevalent. Finally, life sciences companies continued in 2011 to have relative success in obtaining dismissals of securities fraud class action lawsuits brought in past years.
In this survey, we first highlight trends from the securities fraud lawsuits filed against life sciences companies in 2011, including a discussion of some of the notable allegations made in those suits. We then analyze the status of securities fraud lawsuits filed in 2007 through 2010. We next look at what impact the U.S. Supreme Court's March 22, 2011 decision in Matrixx Initiatives, Inc. v. Siracusano — a decision of direct applicability to publicly traded life sciences companies — had on life sciences securities fraud class actions during 2011. Finally, we provide guidance that may help minimize or eliminate the risk of securities fraud class action lawsuits.
Findings
The Numbers
There were 17 securities fraud class action lawsuits brought against life sciences companies in 2011, as compared to a total of 188 securities fraud class action lawsuits brought against all companies in the same time period.1,2 Hence, approximately 9% of the 2011 cases were brought against life sciences companies. 2011, therefore, witnessed a decline in securities fraud class action lawsuits against life sciences companies both from a gross perspective (29 lawsuits in 2010) and from a relative perspective (16% in 2010). This year's proportion of securities fraud class actions brought against life sciences companies is closer to, but still below, the percentage of securities fraud complaints filed against life sciences companies in the years preceding 2010 (10% in 2009, 10% in 2008, 14% in 2007, 13% in 2006).
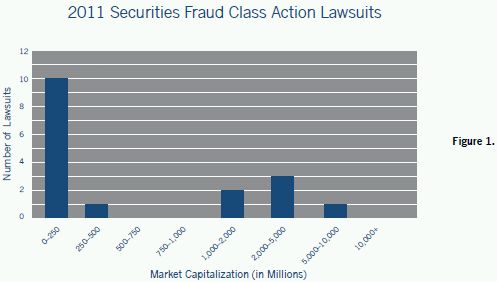
The securities fraud complaints filed in 2011 also focused more on the life sciences companies with relatively smaller market capitalizations (see Figure 1). Whereas in 2010 only 31% of life sciences companies sued for securities fraud had a market capitalization of less than $250 million, this past year 58% of the securities fraud class action lawsuits were brought against companies with market capitalizations of less than $250 million. Additionally, while 28% of such lawsuits in 2010 were filed against the life sciences companies with the market capitalizations of more than $10 billion, no lawsuit was filed against such a company in 2011.
The Nature of the Claims
In a continuing nod to the "Great Recession," the allegations in the securities fraud complaints against life sciences companies continue to display a heavy focus on the company's alleged financial improprieties. The proportion of accounting impropriety allegations, however, has diminished. In 2010, over half of the complaints against life sciences companies containing allegations of financial improprieties; in 2011, such claims were asserted in just 35% of the complaints filed (see Figure 2). Overall, there was a noticeable shift back to more industry-specific allegations in 2011, with a specific focus on allegations of improper marketing practices.

Allegations of misstated or misleading financial results still were common in 2011. For example, in July 2011, China Medicine, a distributor of prescription and over-the-counter medicine in China, along with its accounting firm and certain of its officers, was sued in the Central District of California based on allegations that it had issued incorrect and unreliable financial statements for the years 2006–2009. When the company announced it would be restating its financial results, the stock price plummeted and the shareholders filed suit.3
Similarly, Medifast Inc., a diet company that manufactures, distributes, and sells weight-loss food products and dietary supplements, was sued in September 2011 in the District Court for the District of Maryland. Plaintiffs allege that Medifast lacked proper internal controls to ensure that its financial statements were accurate. When Medifast announced that it would need an extension to file its year-end results for 2010, the stock price fell 24%. Medifast later restated its financial statements for the years ending December 31, 2008 and 2009, indicating that earnings-per-share previously had been overstated. This disclosure resulted in a further decrease in stock value, prompting the lawsuit.
Industry-specific allegations were comparatively on the rise in 2011. Most significant is the number of securities fraud lawsuits arising out of alleged fraudulent marketing of life sciences products. For example, K-V Pharmaceuticals, a company that develops, manufacturers, acquires, and markets branded and generic pharmaceuticals, was sued in the Eastern District of Missouri for fraudulent marketing. The company obtained orphan status for the drug now known as Makena from the FDA and advertised that the drug would be made available "to every eligible patient." However, the 1490% price increase for the drug from the generic version prevented the company from fulfilling this promise. When two senators issued press releases to the FTC disclosing the advertisement to be fraudulent, the stock price fell 14% and company shareholders brought suit.
Similarly, shareholders of Radient Pharmaceuticals brought a securities fraud class action lawsuit in the Central District of California based on allegations of fraudulent marketing. Radient specializes in the research and development of an in-vitro diagnostic cancer test called Onko-Sure. While developing Onko-Sure, Radient began advertising that it had entered into a partnership agreement with the Mayo Clinic to conduct clinical studies, which attracted interest among investors. When it was publicly revealed that no such agreement existed, the stock price fell 26%, prompting the lawsuit.
Several other lawsuits highlighted traditional claims of allegedly fraudulent statements regarding the safety and efficacy of life sciences products. For example, Human Genome Sciences Inc. developed the drug "belimumab," also known as "Benlysta," for the treatment of a certain form of lupus. In various public statements, the company promoted the safety and efficacy of the drug, based on a series of clinical trials. The company did not disclose that several of the patients receiving the drug in the trials committed suicide, whereas no patient receiving the placebo did the same. The stock price fell when the FDA expressed concerns about the clinical trials because of these undisclosed suicides.
Other industry-specific claims involved fraudulent statements regarding prospects for FDA approval. Shareholders brought suit against Mannkind Corp., a developer of the diabetes drug AFREEZA, in the Central District of California. Mannkind had developed an inhaler to deliver the drug but the original design was too expensive to mass produce and too complicated for customers to use. Mannkind opted to develop a new design but sought FDA approval based on clinical trials conducted for the original design, asserting that the two designs were "bioequivalents." Mannkind assured investors that FDA approval was imminent because of the success of the original clinical trials. The stock price fell, however, when the FDA expressed concerns regarding the validity of the original clinical trials and, accordingly, refused to approve the new device.
The Status of Cases Filed Since 2007
The relative success (or failure) of securities fraud class actions filed against life sciences companies is an important data point for consideration. Accordingly, we have reviewed the status of all the securities fraud class action lawsuits filed against life sciences companies since 2007. See Figure 3 for a report on the status of those cases.

As we have noted in our previous surveys, courts will not accept a plaintiff's vague or conclusory allegations against a life science company in lieu of the detailed pleading requirements of the Private Securities Litigation Reform Act ("PSLRA"). In 2011, courts continued to grant with relative frequency life sciences companies' motions to dismiss due to plaintiffs' inability to sufficiently plead scienter. For example, in September 2011, a judge in the District Court of Massachusetts dismissed a complaint against Boston Scientific Corporation because the plaintiffs had failed in their pleadings to establish an adequate inference of scienter.4 The District Court for the Northern District of California dismissed a complaint against XenoPort for the same reason.5
As a warning, it is also worth noting that, even in cases that are settled, securities fraud class action lawsuits can result in very large payments. For example, in 2011, the class action lawsuit against Addus Homecare, filed in 2010 in the Northern District of Illinois, was settled for $3 million.6
Expectations for the Future
In our last survey, we discussed the U.S. Supreme Court's March 2011 decision in Matrixx Initiatives, Inc. v. Siracusano, --- U.S. ----, 131 S.Ct. 1309 (March 22, 2011). In that noteworthy case, Matrixx's shareholders alleged that the company violated the antifraud provisions of Section 10(b) of the 1934 Securities Exchange Act and Rule 10b-5 promulgated thereunder when it failed to disclose that one of its over-the-counter products, Zicam Cold Remedy, caused a loss of smell ("anosmia"). According to plaintiffs, despite evidence that Zicam caused loss of smell in its users, such as contained in "adverse event reports"7 filed about the product, Matrixx continued to issue false and misleading statements that Zicam was safe until 2004, when it stated in a press release that there was "insufficient scientific evidence" regarding the issue.
Of particular importance to life sciences companies, the Supreme Court provided guidance on the question of whether a publicly traded life sciences company can be held liable for securities fraud for failing to disclose adverse event reports regarding its products. With regard to the materiality inquiry, the Court rejected Matrixx's argument that "adverse event reports that do not reveal a statistically significant increased risk of adverse events from product use are not material information."8 At the same time, the Court was careful to explain that statistical significance (or the lack thereof) was not irrelevant.9 Companies were not subject to an affirmative duty to "disclose any and all information."10 The Basic v. Levinson, 485 U.S. 224 (1988), standard of materiality still requires the plaintiff to show that "there is a substantial likelihood that the disclosure of the omitted fact would have been viewed by the reasonable investor as having significantly altered the 'total mix' of information made available."11 Rather than adopting a pure bright-line rule on statistical significance, Matrixx requires the plaintiffs to show "something more."12 With regard to the scienter inquiry, the Court rejected any bright-line test that requires "an allegation of statistical significance to establish a strong inference of scienter."13
In our last survey, we noted that, post-Matrixx, life sciences companies are now faced with the challenging and heavily fact-specific task of determining where to draw the disclosure line in the absence of a bright-line standard. For example, the District Court of New Jersey applied the Supreme Court's materiality holding in Matrixx in a securities fraud class action litigation alleging Section 10(b) violations related to the cardiovascular safety profile of Vioxx, a prescription arthritis drug marketed by Merck.14 Plaintiffs, shareholders of the company, claimed that Merck had overstated the commercial prospects of the drug by downplaying an alleged link between Vioxx and certain cardiovascular events, most notably heart attacks.15 Plaintiffs claimed that defendants had evidence that Vioxx was linked with cardiovascular events even before it was introduced into the market.16 In its decision, the District Court reiterated that, according to Matrixx: "mere adverse event data does not necessarily alter the total mix of information about the drug's safety, as such information by itself does not shed light on whether the drug in question is causing the event."17 Instead, the materiality standard requires a "fact-intensive, contextual inquiry."18 Whether "non-disclosure [is] actionable under the securities laws [is] very much a function of what information a company had chosen to communicate to the market."19 In that case, applying the reasoning set forth in Matrixx, the District Court found that plaintiffs had adequately pleaded a material misrepresentation with respect to statements made by the company touting Vioxx's safety.20 Significantly, the District Court found that the "non-disclosed information [went] beyond adverse event reports."21 Such information included internal company data that showed that patients taking Vioxx experienced more cardiovascular events than patients taking a placebo, women taking the drug had more than twice as many cardiovascular events as women taking a placebo, and a clinical trial conducted prior to FDA approval suggested the existence of a possible causal relationship between the drug and cardiovascular events.22 Accordingly, the Court rejected defendants' argument that the "lack of data supporting a conclusive link between Vioxx and heart attacks precludes the undisclosed information from meeting the materiality standard."23
The Southern District of New York also followed the materiality holding of Matrixx in a class action securities fraud lawsuit filed by the shareholders of Sanofi-Aventis SA in connection with the company's anti-obesity drug rimonabant.24 In that case, plaintiffs alleged that the company failed to disclose adverse event data during clinical study that it had provided upon request to the FDA, which showed the drug's possible link to suicidality.25 In denying sanofi-aventis' motion to dismiss, the District Court squarely applied Matrixx to reject defendants' arguments that the company had no duty to disclose because the adverse events were not statistically significant, and that the alleged omissions were immaterial.26
Thus far, Matrixx has not resulted in any noticeable increase in securities fraud lawsuits brought against life sciences companies. Although, as noted above, there has been some limited application of the Court's holding in addressing materiality, the scienter aspect of Matrixx has not yet shown any significant impact on existing case law beyond rejection of the bright-line rule based on lack of statistical significance.
Minimizing the Risk of Securities Fraud Class Actions
There are several steps that life sciences companies can take to reduce the risk of, or impact from, securities fraud class actions. Aside from the obvious strategy of ensuring that the companies' statements and public filings are truthful and accurate, the following should be considered:
- Be alert to events that may negatively impact the drug product lifecycle. Some potentially troubling issues are obvious, e.g., clinical trial failures and FDA rejection. Others, however, are not so obvious, such as manufacturing problems, the loss of a key commercial partner, or an increased percentage of revenues being derived from off-label uses.
- In light of the Matrixx decision, review internal processes relating to communications and disclosure about products, including those that are not yet on the market.
- Develop and publish employee guidelines tailored to specific areas of business operations. Communications by the R&D and marketing departments become subject to particular scrutiny in securities fraud lawsuits filed against life sciences companies.
- Ensure that the public statements and filings contain appropriate "cautionary language" or "risk factors" that are specific and meaningful, and cover the gamut of risks throughout the entire drug product life cycle — from development to production to commercialization.
- Ensure that the sometimes fine line between puffery and statements of fact is not crossed in public statements or filings, or even in extemporaneous statements during analyst calls and media commentary. While soft puffery contains a positive message and image about a company that is not misleading under securities laws, it is upon hard statements of fact that class action lawyers — with the benefit of 20/20 hindsight — will concoct a lawsuit.
- Develop and publish an insider trading policy to minimize the risk of inside trades during periods that might help class action lawyers later develop a theory. Class action lawyers aggressively monitor trades by insiders to develop allegations that a company's executives knew "the truth" and unloaded their shares before it was disclosed to the public and the stock plummeted.
Footnotes
1 The number of securities fraud class actions brought against life sciences companies, as well as the total number of securities fraud class actions, is based on information reported by the Securities Class Action Clearinghouse in cooperation with Cornerstone Research.
2 Securities fraud class actions filed in the context of a merger or other change in control transaction are not included in this Survey despite being generally considered on the rise. See M&A Related Litigation Has Replaced Stock Drop Suits as Plaintiffs' Securities Lawyers' Lawsuit of Choice (last accessed 3/5/2012).
3 Significantly, half of the claims alleging financial improprieties were brought against China-based companies. This trend is consistent with patterns noticed in all securities class actions in 2011. See Cornerstone Research, Securities Class Action Filings: 2011 Year in Review, at *1.
4 In re Boston Scientific Corp. Sec. Litig., No. 10-10593, 2011 U.S. Dist. LEXIS 106042, at *45-6 (D. Mass. Sept. 19, 2011).
5 In re Xenoport, Inc. Sec. Litig., No. C-10-03301, 2011 U.S. Dist. LEXIS 142523, at *20 (N.D. Cal. Dec. 12, 2011).
6 Crotteau v. Addus Homecare Corp., No. 10-C-1937 (N.D. Ill. 2010) (Notice of Proposed Settlement).
7 An adverse event is "any undesirable experience associated with the use of a medical product in a patient." See "What is a Serious Adverse Event?" (last visited February 13, 2011).
8 Matrixx Initiatives, Inc. v. Siracusano, 131 S.Ct. 1309, 1318 (March 22, 2011).
9 Id. at 1321.
10 Id.
11 Basic, 485 U.S. at 231-32.
12 Matrixx, 131 S. Ct. at 1321.
13 Id. at 1313.
14 In re Merck & Co., Inc. Securities, Derivative & ERISA Litig., 2011 U.S. Dist. LEXIS 87578, at *26-7 (D.N.J. Aug. 8, 2011).
15 Id. at *28.
16 Id.
17 Id. at *58.
18 Id. at *59.
19 Id.
20 Id. at *60.
21 Id. at *58.
22 Id. at *60-61.
23 Id.
24 In re Sanofi-Aventis Securities Litig., 774 F. Supp. 2d 549 (Mar. 30, 2011).
25 Id. at 558.
26 Id. at 563.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

