An Advisory Group of 29 scientists from 18 countries met in March, 2019, to recommend priorities for the International Agency for Research on Cancer (IARC) Monographs programme during 2020–24. IARC periodically convenes such advisory groups to ensure that the Monographs evaluations reflect the current state of scientific evidence relevant to carcinogenicity.1 A detailed report of the Advisory Group will be published subsequently. 2
The Advisory Group assessed the response to a public call for nominations and considered more than 170 unique candidate agents, including the recommended priorities remaining from a similar Advisory Group meeting convened in 2014. 3 The expertise of the Advisory Group covered multiple disciplines, and the members appraised, on an individual nomination basis, the evidence according to human exposure (including any evidence of exposure in low-income and medium-income countries), cancer epidemiology, cancer bioassays in experimental animals, and carcinogen mechanisms, in line with the evaluation methodology recently refined in the Preamble to the IARC Monographs. 1 A complementary approach assessed all nominations using a chemoinformatics, text mining, and chemical similarity analysis workflow; 4 this approach helped to reveal coverage and gaps in the extent of evidence across data streams, supporting decisions on individual agents and groups of chemically related nominations. The Advisory Group deliberated on all nominated agents both by evidence stream (ie, exposure, human cancer, cancer bioassay, and carcinogen mechanisms) and by type of agent (eg, metals, fibres, chemicals, biological agents, and complex mixtures) to inform development of priority recommendations.

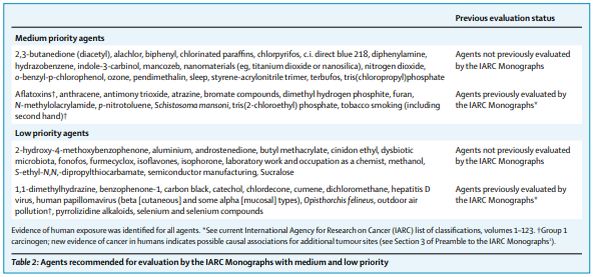
The Advisory Group recommended a broad range of agents with high (table 1), medium, or low (table 2) priority for evaluation. Priority was assigned on the basis of evidence of human exposure and the extent of available evidence for evaluating carcinogenicity (ie, the availability of relevant human cancer, experimental animal bioassay, or mechanistic evidence to support a new or updated evaluation according to the Preamble to the IARC Monographs1 ). Any of the three evidence streams could alone support prioritisation of agents with no previous evaluation. For previously evaluated agents, the Advisory Group considered the basis of the previous classification, as well as the potential impact of the newly available evidence during integration across streams (see table 4 in Preamble to the IARC Monographs1 ). Agents without evidence of human exposure or evidence for evaluating carcinogenicity were not recommended for further consideration. The Advisory Group recognised that agents related to the identified priorities might also warrant evaluation. Furthermore, additional agents might merit consideration if new relevant evidence indicating an emerging carcinogenic hazard (eg, from cancer epidemiology studies, cancer bioassays, or studies on key characteristics of carcinogens) becomes available in the next 5 years. In line with the interim standard operating procedure adopted by the IARC Governing Council,5 IARC will consider this advice when selecting agents for future Monograph evaluations according to the Preamble to the IARC Monographs. 1
Footnote
1 International Agency for Research on Cancer. Preamble to the IARC Monographs. 2019. https://monographs.iarc.fr/wp-content/ uploads/2019/01/Preamble-2019.pdf (accessed April 16, 2019)
2 International Agency for Research on Cancer. Report of the Advisory Group to Recommend Priorities for IARC Monographs during 2020–2024; 25–27 March, 2019. Lyon: Monographs on the Evaluation of Carcinogenic Risks to Humans, in press
3 Straif K, Loomis D, Guyton K, et al. Future priorities for the IARC Monographs. Lancet Oncol 2014; 15: 683–84.
4 Guha N, Guyton KZ, Loomis D, Barupal DK. Prioritizing chemicals for risk assessment using chemoinformatics: examples from the IARC Monographs on pesticides. Environ Health Perspect 2016; 124: 1823–29.
5 International Agency for Research on Cancer. Sixtieth Session of the IARC Governing Council, GC/60/13. 2018. http://governance.iarc.fr/GC/ GC60/En/Docs/GC60_13_CoordinationWHO. pdf (accessed April 12, 2019).
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.
