WHO urges governments to take action
An estimated 1-in-10 medical products circulating in low- and middle-income countries is either substandard or falsified, according to new research from WHO1.
WHO said that this means that people are taking medicines that fail to treat or prevent diseases. Not only is it a waste of money for individuals and health systems that purchase these products, but substandard or falsified medical products can cause serious illness or even death.
WHO further said that since 2013 it has received 1500 reports of cases of substandard or falsified products. Of these, anti-malarials and antibiotics are the most commonly reported, followed by Lifestyle products that include products for cosmetic use, erectile dysfunction, body-building and dieting.
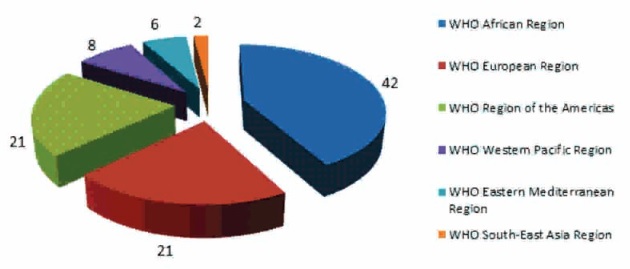
About 42% of the reports come from the WHO African Region, 21% from the WHO Region of the Americas, and 21% from the WHO European Region. WHO also said that this is likely just a small fraction of the total problem and many cases may be going unreported. For example, only 8% of reports of substandard or falsified products to WHO came from the WHO Western Pacific Region, 6% from the WHO Eastern Mediterranean Region, and just 2% from the WHO South-East Asia Region (Figure 1 below).
The WHO research highlights that it has received reports of substandard or falsified medical products ranging from cancer treatment to contraception. These are not confined to high-value medicines or well-known brand names but are split almost evenly between generic and patented products.
In conjunction with the first report from the Global Surveillance and Monitoring System2, WHO has also published a research that estimates a 10.5% failure rate of all medical products used in low- and middle-income countries.
This study was based on more than 100 published research papers on medicine quality surveys done in 88 low-and middle-income countries involving 48000 samples of medicines. Lack of accurate data means that these estimates are just an indication of the scale of the problem. More research is needed to accurately estimate the threat posed by substandard and falsified medical products.
Based on the 10% estimates of substandard and falsified medicines, a modelling exercise developed by the University of Edinburgh estimates that 72000 to 169000 children may be dying each year from pneumonia due to substandard and falsified antibiotics. A second model developed by the London School of Hygiene and Tropical Medicine estimates that 116000 (64000 – 158000) additional deaths from malaria could be caused every year by substandard and falsified anti-malarials in sub-Saharan Africa, with a cost of US$ 38.5 million (21.4 million – 52.4 million) to patients and health providers for further care due to failure of previous treatment.
What are substandard and falsified medical products?
Substandard medical products
- Also called "out of specification", these are authorized medical products that fail to meet either their quality standards or their specifications, or both3.
Unregistered/unlicensed medical products
- Medical products that have not undergone evaluation and/or approval by the NMRA for the market in which they are marketed/distributed or used, subject to permitted conditions under national or regional regulation and legislation.
Falsified medical products
- Medical products that deliberately/fraudulently misrepresent their identity, composition or source4.
The Internet Gateway
WHO also reported that in some high-income countries, medical products bought over the Internet from illegal or unauthorized websites, social media platforms or smartphone applications frequently fail to meet quality standards. Online pharmacies have become increasingly popular, for example in the United States of America, the number of people buying medicines online has more than quadrupled in less than a decade52. The inexorable growth in online sales provides criminals with a relatively easy entry point into even the best regulated markets. Authorities around the world are working to tackle this new challenge, but it is universally recognized to be a difficult task. Modern purchasing models such as online pharmacies can easily circumvent regulatory oversight. Regulating the supply of medicines and investigating the online supply of substandard and falsified medical products is complex, often involving several countries. This can lead to jurisdictional complexities and the requirement of evidence from multiple countries.
Substandard and Falsified Medical Products: The Solution
According to the WHO report, Globalization is making it harder to regulate medical products. Many falsifiers manufacture and print packaging in different countries, shipping components to a final destination where they are assembled and distributed. Sometimes, offshore companies and bank accounts have been used to facilitate the sale of falsified medicines.
Therefore, WHO has called for serious, well-resourced efforts to tackle the issue and has summarized what needs to be done to achieve this:
- PREVENT the manufacture, sale and consumption of substandard and falsified medical products;
- implement systems to DETECT any substandard or falsified products that are already in the supply chain;
- RESPOND quickly and proportionately to any incidents that are detected, in ways that safeguard patients and the supply chain, take appropriate action against those responsible, whilst not causing unnecessary shortages.
Most of these actions require the coordinated participation of a number of different actors, including national and regional governments; global organizations; the private and non-profit sectors; and civil society. Effective action also requires close collaboration between disciplines: health authorities must work with customs and law enforcement agencies; pharmacovigilance systems must link to those that track antimicrobial resistance and falsified products; pharmaceutical and logistics companies must exchange information with regulators; patient and consumer groups must interact fluently with authorities.
Conclusion
Substandard medical products reach patients when the tools and technical capacity to enforce quality standards in manufacturing, supply and distribution are limited. Falsified products, on the other hand, tend to circulate where inadequate regulation and governance are compounded by unethical practices by wholesalers, distributors, retailers and health care workers. A high proportion of cases reported to WHO occur in countries with constrained access to medical products.
WHO has called this a global problem, and calls on nations for a globally coordinated response to tackle the issue. Countries need to assess the extent of the problem at home and cooperate regionally and globally to prevent the traffic of these products and improve detection and response.
Footnotes
1 http://www.who.int/mediacentre/news/releases/2017/substandard-falsified-products/en/
2 http://www.who.int/entity/medicines/regulation/ssffc/publications/gsms-report-sf/en/index.html
3 Appendix 3 to Annex, World Health Assembly document A70/23, 2017.
4 WHO Global Surveillance and Monitoring System for substandard and falsified medical products, 2017
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

