Overview of Qui Tam Activity
- We identified 46 health care related qui tam cases that were unsealed in February and March 2018.
- The government intervened in whole or in part in 14% of those unsealed cases, which was identical with the prior two-month period and is consistent with the overall intervention rate during the prior 12 months.
- Of the 46 unsealed cases, only 17 were dismissed in their entirety. The other 29 remained active.
- The 46 unsealed cases were filed in 38 different courts. Jurisdictions with the most unsealed cases were the Middle District of Florida (Jacksonville, Orlando, and Tampa) with six, the Northern District of Ohio (Cleveland) with three, and the District of Kansas, the Eastern District of Tennessee (Chattanooga and Knoxville), the Northern District of Illinois (Chicago), and the Southern District of Florida (Miami, Fort Lauderdale, and West Palm Beach) with two apiece.
- Home health and hospice providers were defendants in seven of the cases. Seven cases were also brought against hospitals and hospital systems, and five cases were brought against pharmaceutical and biotech firms. Three of the unsealed cases named physicians or physician practice groups, while another three were brought against health insurers.
- As is typically the case, former employees were again the most frequent relator type, accounting for 23 of the 46 unsealed cases. Current employees only brought three of the cases. Three cases were brought by defendants' business partners.
- None of the cases was unsealed within the 60-day period specified by statute, although one was unsealed after 61 days. The longest time under seal was just over six years. The average time under seal for this group of unsealed cases was 851 days. Only five of the 46 unsealed cases were unsealed in less than one year.
Featured Cases
United States ex rel. Cleary v. Ioannides, No. 2:15-cv-14306-RLR (S.D. Fla.)
Complaint Filed: August 31, 2015
Complaint Unsealed: March 7, 2018
Intervention Status: The government intervened on December 4, 2017, for purposes of settlement.
Claims: False Claims Act ("FCA"), 31 U.S.C. § 3729 et seq.
Defendants' Businesses: Defendant is a dermatologist who practices through Tim Ioannides, M.D., LLC d/b/a Treasure Coast Dermatology, which is also a named defendant.
Relator: Patricia Cleary
Relator's Relationship to Defendants: The relator was a patient of Dr. Ioannides and Treasure Coast Dermatology.
Relator's Counsel: Iain Leslie Cooper Kennedy, Paul Thomas Reid, and Timothy Michael Moore of Shook, Hardy & Bacon L.L.P. and Theodore Charles Miloch, II of Infante Zumpano Hudson & Miloch LLC
Summary of Case: The relator, a patient of the defendant Ioannides, alleged that Ioannides billed and received payment from Medicare for a procedure he did not perform on her. Specifically, the relator alleged that she saw Ioannides, a dermatologist, for removal of a potentially cancerous lesion from her forehead. However, Ioannides billed the procedure as flap surgery, a procedure performed by plastic surgeons that involves moving a muscle and skin flap from a donor site to a recipient site without disconnecting the flap's existing blood supply. The procedure is commonly performed on patients who have received mastectomies and cannot be performed on a patient's forehead. The relator also contended that Ioannides committed this same fraud numerous times beginning at least as early as 2012. Citing information obtained from the publically available CMS Medicare database, the relator asserted that Ioannides was the country's top biller for flap surgeries in 2012 and 2013. The relator charged that Ioannides violated the FCA by fraudulently billing Medicare for flap surgeries she claimed he had not performed.
Current Status: The United States intervened on December 4, 2017, and entered into a Settlement Agreement with the defendants on December 21, 2017. Pursuant to this Settlement Agreement, defendants were to pay $2,500,000 to the United States. The settlement amount was payable in installments, due in full by January 31, 2018. The relator received 19% of the total settlement amount. The complaint was dismissed and unsealed in part on March 7, 2018.
Reasons to Watch: This is an interesting case in several respects. It is relatively rare for patients to serve as relators, but the number of patient relators has been growing in recent years. It is also interesting to see a large defense-oriented law firm, Shook Hardy, serving as one of the relator's lawyers. But possibly the most interesting aspect of this case is the use of data from the CMS Medicare database to support the relator's claims. A 1979 injunction prohibited CMS from disclosing physician-identifying data. In 2013, however, that injunction was lifted, making it possible to link Medicare claims data to physicians who performed the services. This case provides an example of how relators can make use of that data to attempt to demonstrate the extent of allegedly fraudulent billings by physicians alleged to have violated the FCA. It is likely that growing numbers of relators will mine the CMS Medicare database for similar patterns of activity in order to support qui tam litigation.
United States ex rel. Knopf v. Agevital Pharmacy, LLC, No. 8:15-cv-02591-CEH-JSS (M.D. Fla.)
Complaint Filed: November 4, 2015
Complaint Unsealed: March 9, 2018
Intervention Status: On March 8, 2018, the government entered a notice that it is not intervening at this time.
Claims: FCA
Defendants' Businesses: Defendant AgeVital Pharmacy, LLC, is a compounding pharmacy that provides compounded pharmaceuticals to Medicare beneficiaries, among other customers. Defendant Jean Wilson, FNP-BC, is a nurse practitioner licensed in Maryland, New Jersey, New York, and North Carolina.
Relator: Manfred Knopf
Relator's Relationship to Defendants: The relator allegedly received unsolicited compounded pharmaceuticals that he neither wanted nor needed from AgeVital.
Relator's Counsel: Sean Keefe and Elaine Stromgren of James Hoyer, P.A., David Caputo and David Williams of Kline & Specter, P.C., and Claudine Q. Homolash of CQH Firm.
Summary of Case: The relator resides in New Jersey. In mid-2014, he slipped and suffered an injury that caused him pain. Between July and December 2014, relator sought and received treatment from several providers for his injury. The relator alleged that he received at least three unsolicited phone calls from AgeVital between August and December 2014. These callers were aware of the relator's medical history, requested his insurance information and date of birth, and stated they wanted to help him. Ultimately, the relator received three shipments containing two compounded pharmaceuticals. The prescriber listed on the containers was "Dr. Jean Wilson," yet the relator alleged that he had never heard of this person nor had he been treated by someone named "Dr. Jean Wilson." The NPI number associated with "Dr. Jean Wilson" belongs to Ms. Wilson, a nurse practitioner with a business mailing address in Bayonne, New Jersey. The relator asserted that he neither requested nor sought these treatments and that Medicare paid AgeVital over $37,000 for the compounded pharmaceuticals. The relator asserted that he neither requested nor sought these treatments and that Medicare paid AgeVital over $37,000 for the compounded pharmaceuticals. He also charged that the described solicitation and billing practices were fraudulent and that the defendants conspired to submit false claims to the federal government in violation of the FCA.
Current Status: In early March 2018, the United States decided not to intervene "at this time."
Reasons to Watch: This is another case in which the relator is a patient. In this particular case, the FCA allegations arose from alleged fraud committed not only against the government but also against the patient-relator. This case typifies the increasing use of the FCA to target common law fraud.
United States ex rel. Kaplan v. Northern Metropolitan, Inc., No. 16-cv-2180-ALC (S.D.N.Y.)
Complaint Filed: March 24, 2016
Complaint Unsealed: March 8, 2018
Intervention Status: Intervention by the United States on March 7, 2018
Claims: FCA; State of New York False Claims Act, New York Finance Law, § 187 et seq.
Defendants' Businesses: Defendant Northern Metropolitan, Inc. ("NMET") is a residential health care facility located in Monsey, New York and a wholly owned subsidiary of defendant NCH Healthcare System, Inc. Defendant Northern Riverview Health Care Center, Inc. ("NRV") is a Section 501(c)(3) entity, which is the operator of Riverside Nursing Home. Defendant Northern Services Group, Inc. ("NSG") is a shared services parent organization created to coordinate the elder care of Chevre Liady Nusach Hoary. Defendant Northern Metropolitan Foundation for Healthcare, Inc. ("NMFHC") is a Section 501(c)(3) entity, which replaced NSG as the corporate member of NMET and NRV. Defendant Chevre Liady Nusach Hoary ("CLNH") is a not-for-profit religious organization, which is the owner of NMET.
Relator: Sonny Kaplan
Relator's Relationship to Defendants: Mr. Kaplan was a full-time financial employee of NRV beginning on January 10, 2000.
Relator's Counsel: Philip Roy Michael of Michael Law Group and Mark H. Schwartz of the Law Office or Mark H. Schwartz.
Summary of Case: The relator alleged that his superiors at NRV, several years after being appointed by the New York State Department of Health ("DOH") as the receiver of Riverside Nursing Home, became aware of a double-counted Medicaid Transfer Price ("MATP") of $2,180,000, totaling $4,360,000. A MATP consists of reimbursement, paid annually, over a period of 20 or 30 years, until the full amount of un-depreciated capital costs incurred by an acquiring health care provider is fully repaid. The relator alleged that his superiors at NRV were aware of the double-counted MATP but nonetheless retained the double-counted MATP, which was being paid year-to-year to NRV. The relator generally asserted that no NRV accountant or operator informed the DOH or federal government of the double-counted MATP. The relator also alleged that beginning in 1998, as a result of the error in the MATP calculation, Medicaid made excessive depreciation-reimbursement payments to NRV. In addition, the relator contended that his superiors at NRV informed him of their justification for failing to disclose the error to DOH or the federal government, suggesting that because of a systemic unfairness in the manner in which DOH reimbursed facilities, the error resulted in a balanced reimbursement to NRV.
Current Status: On March 7, 2018, the United States intervened in the case, and the complaint was unsealed on March 7, 2018. On March 12, 2018, a New York State Stipulation and Order of Settlement and Dismissal and a federal Stipulation and Order of Settlement and Dismissal were entered by the Court. In the stipulations, the defendants admitted to reporting erroneous capital costs of $2,183,679, resulting in "hundreds of thousands of dollars" in overpayments to the defendants. Under the settlement, the defendants paid an aggregate amount of $500,000 to the federal government and the State of New York, with $288,313.82 to be paid to New York and $211,686.18 to be paid to the United States. The relator's share of the New York settlement was $51,896.49, and the United States paid the relator $42,337.24.
Reasons to Watch: Kaplan provides insight into the risks that a party may face in failing to provide authorities with voluntary disclosure of known overpayment amounts. The case illustrates a scenario where a medical services provider intentionally concealed a known repayment obligation, only to have their unlawful conduct reported by a former employee.
United States ex rel. Kelly v. Bromedicon, Inc., No. 2:13-cv-7635-TJS (E.D. Pa.)
Complaint Filed: December 30, 2013
Complaint Unsealed: March 13, 2018
Intervention Status: Intervention by the United States on March 8, 2018.
Claims: FCA
Defendants' Businesses: Bromedicon, Inc. ("Bromedicon") is a corporation, established in 1986 and owned by James Brogan ("Brogan"). Bromedicon primarily engages in the provision of IntraOperative Neurophysiological Monitoring ("IOM"). IOM involves the remote monitoring, by a qualified medical professional (known as a "qualified interpretation professional" or "QIP"), of a patient's neurological status and neural structures during surgery to assist in the prevention of damage to that patient's nervous system. Bromedicon provides the service to over 3,000 patients per year.
Relators: Dianna Kelly
Relator's Relationship to Defendants: Ms. Kelly was employed by Defendant as an office manager.
Relator's Counsel: Sidney L. Gold of Sidney L. Gold & Assoc. P.C.; Neelima Vanguri of Sidney L. Gold & Assoc. P.C.
Summary of Case: The relator alleged that Bromedicon billed Medicare for IOM services that were never provided. The relator claimed that Bromedicon provided IOM services without necessarily having QIPs available to perform all contracted monitoring services. Where a QIP was unable to monitor a procedure, Bromedicon purportedly had a QIP sign a fabricated interpretation report associated with the procedure that represented to Medicare that the IOM service was, in fact, provided to the patient. The relator further alleged that Bromedicon billed for more IOM services than were appropriate, or even possible, given scheduling and staffing constraints. The relator also asserted that Bromedicon inflated the time that QIPs spent performing IOM services and billed Medicare for the full – inflated – time. The relator claimed to have informed Bromedicon about these practices, but Bromedicon failed to report the relator's findings to the Office of the Inspector General of the Department of Health and Human Services ("OIG") or the Department of Justice ("DOJ").
Current Status: On March 8, 2018, the United States intervened in the case. The complaint was unsealed five days later on March 13, 2018. A subsequent sealed order entered on April 26, and no further proceedings have occurred on the public record.
Reasons to Watch: Kelly highlights the potential risks associated with providing patient services remotely. As telemedicine becomes more prevalent, there is an increased risk of actual – or claimed – failure to provide remote services. Providers that provide services remotely need to develop good systems and practices to ensure accountability by remote providers, both to avoid fraud and to defend against fraud claims. And all types of providers should be mindful of the risk, as alleged here, that failure to act on an employee's reporting of potential fraud and abuse can lead to a whistleblower lawsuits.
Health Care Qui Tam Litigation Trends
Mintz Levin maintains a database of unsealed health care qui tam actions. This enables us to follow and analyze trends in the cases that have been unsealed. The following are some trends in qui tam filings against health care-related entities in the 12 months that ended June 30, 2018:
Where were cases filed? Although cases were unsealed in jurisdictions throughout the country, some interesting trends have emerged as to jurisdictions where the most cases have been unsealed:
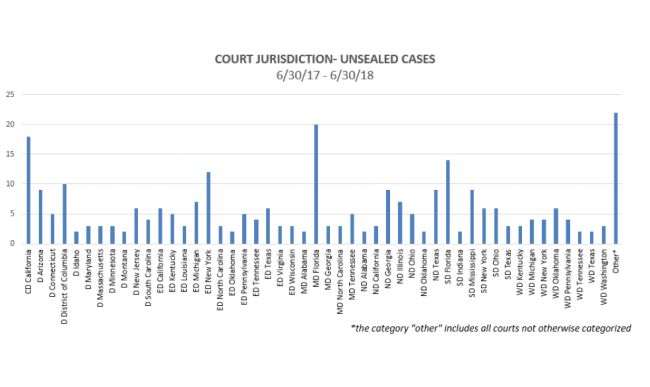
For the 12-month period that ended on June 30, the Middle District of Florida (Tampa, Orlando, and Jacksonville) continued to be the leading jurisdiction for unsealed cases, with 20 cases being unsealed over that time frame. Following closely behind was the Central District of California (including Los Angeles and Santa Barbara), where 17 cases were unsealed. Other courts with 10 or more cases unsealed in that 12-month period were the District of Columbia, the Eastern District of New York (Brooklyn, Queens, and Long Island), and the Southern District of Florida (Miami, Fort Lauderdale, and West Palm Beach).
Who brought the cases? The ranks of relators are beginning to diversify. Current and former employees – mostly the latter – dominate the ranks of relators, accounting for 57% of all cases. But significant numbers of relators are now found among customers, industry experts, business partners, consultants, and patients.
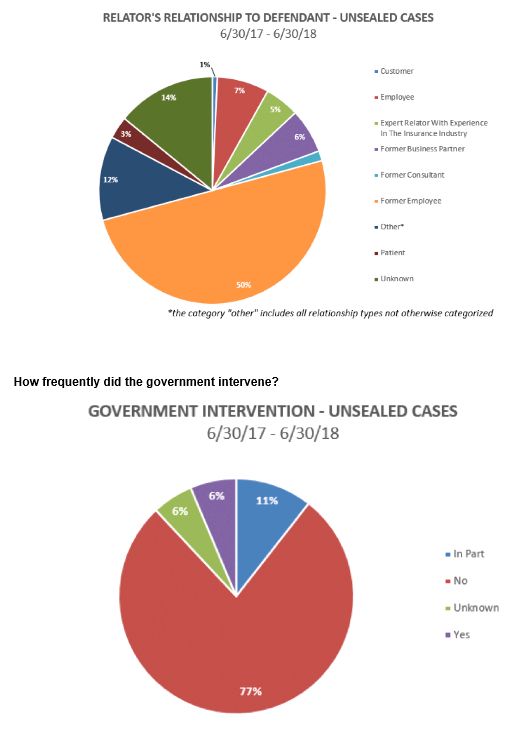
Intervention rates continue to be extremely low, with the government electing to intervene only 17% of cases unsealed in the 12 months that ended June 30, 2018.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.




