Yescarta becomes the second gene therapy product to be approved in the United States
On October 18, 2017, the United States Food and Drug Administration (US FDA) approved Yescarta (Axicabtagene Ciloleucel), a cell-based gene therapy, to treat adult patients with certain types of large B-cell lymphoma who have not responded to or who have relapsed after at least two other kinds of treatments. Yescarta, a chimeric antigen receptor (CAR) T cell therapy, is the second gene therapy approved by the FDA and the first for certain types of non-Hodgkin Lymphoma (NHL)24.
CAR T therapy is a breakthrough in hematologic cancer treatment in which a patient's own T cells are engineered to seek and destroy cancer cells. CAR T therapy is produced uniquely for each individual patient.
Each dose of Yescarta is a customized treatment created by using a patient's own immune system to help fight the lymphoma. The patient's T-cells, a type of white blood cell, are collected and genetically modified to include a new gene that targets and kills the lymphoma cells. Once the cells are modified, they are infused back into the patient.
Diffuse large B-cell lymphoma (DLBCL) is the most common type of NHL in adults. NHLs are cancers that begin in certain cells of the immune system and can be either fast-growing (aggressive) or slow-growing. Approximately 72,000 new cases of NHL are diagnosed in the U.S. each year, and DLBCL represents approximately one in three newly diagnosed cases. Yescarta is approved for use in adult patients with large B-cell lymphoma in whom at least two other kinds of treatments have failed, including DLBCL, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma and DLBCL arising from follicular lymphoma. Yescarta is not indicated for the treatment of patients with primary central nervous system lymphoma.
Commenting on this approval Peter Marks, M.D., Ph.D., Director of the FDA's Center for Biologics Evaluation and Research (CBER) said, "The approval of Yescarta brings this innovative class of CAR-T cell therapies to an additional group of cancer patients with few other options – those adults with certain types of lymphoma that have not responded to previous treatments."
Treatment with Yescarta has the potential to cause severe side effects. It carries a boxed warning for cytokine release syndrome (CRS), which is a systemic response to the activation and proliferation of CAR-T cells causing high fever and flu-like symptoms, and for neurologic toxicities. Both CRS and neurologic toxicities can be fatal or life-threatening. Other side effects include serious infections, low blood cell counts and a weakened immune system. Side effects from treatment with Yescarta usually appear within the first one to two weeks, but some side effects may occur later.
Because of the risk of CRS and neurologic toxicities, Yescarta is being approved with a risk evaluation and mitigation strategy (REMS), which includes elements to assure safe use (ETASU). The FDA is requiring that hospitals and their associated clinics that dispense Yescarta be specially certified. As part of that certification, staff involved in the prescribing, dispensing or administering of Yescarta are required to be trained to recognize and manage CRS and nervous system toxicities. Also, patients must be informed of the potential serious side effects and of the importance of promptly returning to the treatment site if side effects develop.
The FDA granted approval of Yescarta to Kite Pharma, Inc. In August 2017, Gilead Sciences acquired Kite Pharma for 11.9 Billion US dollars.
Yescarta will be manufactured in Kite's state-of-the-art commercial manufacturing facility in El Segundo, California. Kite has demonstrated a 99 percent manufacturing success rate with a median manufacturing turnaround time of 17 days, which is important to patients given the potential for rapid disease progression in this population25.
What is CAR-T therapy?
Chimeric Antigen Receptor T-cell (CAR-T) therapy represents a completely new kind of immunotherapy medicine. CAR-Ts are living cellular biologics—genetically reengineered versions of a patient's own immune cells that have been programmed to recognize and kill cancer cells.
The validation of this novel approach first came at the FDA-hosted Oncologic Drugs Advisory Committee (ODAC) meeting, held in July 2017, to review Novartis's application for the first CAR-T cell therapy. The US Food and Drug Administration's (FDA) Oncologic Drugs Advisory Committee voted unanimously, 10 to 0, in favor of the benefitrisk profile for the first of this new kind of cancer therapy, Chimeric Antigen Receptor T-cell (CAR-T) therapy26. Timothy Cripe, a panel member of Nationwide Children's Hospital in Columbus, Ohio, said that CAR-T is the "most exciting thing I've seen in my lifetime."
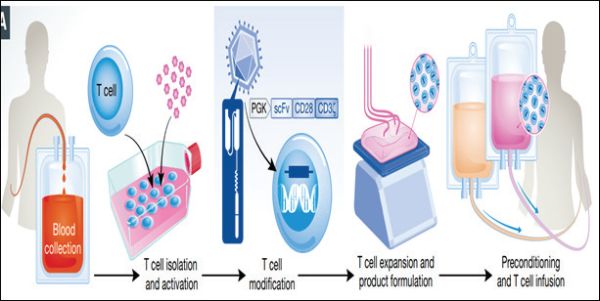
Principle of CAR-T cell therapy process
Individualized CAR-T therapy uses a patient's own immune system to fight certain types of cancers. A patient's T cells are extracted and reprogrammed outside of the body to recognize and fight cancer cells and other cells expressing a particular antigen.
How CAR-T therapy works is presented below27,28:
- Leukapheresis: The process starts with Leukapheresis where a patient's white blood cells, including T cells, are extracted through a specialized blood filtration process (leukapheresis). The T cells are then cryopreserved and sent to manufacturing facility/ laboratory for reprogramming.
- Reprogramming: Using an inactive virus (viral vector), T cells are genetically encoded to recognize cancer cells and other cells expressing a specific antigen.
- Expansion of the T-Cells: Newly created CAR-T cells undergo expansion followed by product formulation.
- Lymphodepleting chemotherapy: Lymphodepleting chemotherapy is given to the patient to reduce the level of white blood cells and help the body accept the reprogrammed CAR-T cells. This process is also called as pre-conditioning.
- Cell Infusion: The reprogrammed CAR-T cells are infused back into the patient's blood.
- Cell Death: Within the patient's body, the CAR-T cells have the potential to recognize the patient's cancer cells and other cells expressing a specific antigen and attach to them, which initiates direct cell death thereby killing the cancer cells.
Conclusion
CAR T therapy is a breakthrough in cancer treatment in which a patient's own T cells are engineered to seek and destroy cancer cells. Engineered cell therapies like Yescarta represent the potential for a changing treatment paradigm for cancer patients who have run out of options of conventional anti-cancer therapies.
Footnotes
24 https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm581216.htm
27 https://novartis.gcs-web.com/static-files/d49ca1ae-3ec9-42bb-bd1a-37441a93e982
28 http://embomolmed.embopress.org/content/embomm/early/2017/07/31/emmm.201607485.full.pdf
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

