In Canada, trademarks for pharmaceutical preparations and related products have faced numerous challenges, some of which are unique to the industry. This article will canvas the current issues as well as the important changes and new opportunities that will be available with the changes to Canadian trademark law that are expected to come into force in 2019.
Current challenges relating to pharmaceutical trademarks
For drug names, pharmaceutical companies need to consider both the requirements of the Trademarks Act as well as Health Canada requirements
If drug names are to be registered as trademarks, those drug name trademarks must obviously comply with the same requirements imposed by the Trademarks Act for any other goods. To be registered as a trademark, among other requirements, the proposed mark must not be confusing with a mark owned by another party. In other words, consumers should not mistakenly believe that the source of two drugs is the same.
Separate from the trademark registration process, drug name approval for prescription pharmaceuticals is required from Health Canada. Importantly, approval of a name by the Trademarks Office does not guarantee approval by Health Canada and vice versa.
Prescription drug names must satisfy a number of criteria to gain approval from Health Canada. One requirement is the need to avoid a situation where relevant members of the public are likely to mistake one drug name for another. Another consideration is how the name will appear in handwriting. If a handwritten rendering of the mark (by a doctor, for example) could be mistaken for another drug name, Health Canada might reject the name.
Declaration of use requirement
If a Canadian trademark application is based on proposed use, that application cannot proceed to registration until a declaration of use (typically confirming sales in Canada) is filed. However, since prescription drugs cannot be sold in Canada without approval from Health Canada and since approval can take many years, owners of trademark applications for drug names are often forced to request extensions of time for several years before they can file a declaration of use.
Challenges when seeking to register the shape/colour of tablets and/or some medical devices
It is possible to register trademarks featuring colour as applied to goods having a specified shape. For most industries, the Trademarks Office and Canadian courts have protected such marks without imposing significant obstacles.
However, that is not the case for shape/colour marks for pharmaceutical tablets, pills, etc. In some decisions, the Federal Court of Canada has ruled that in spite of massive sales, advertising and reputation, the shape/colour of certain tablets is simply not perceived as an indicator of source.
An example is the blue Viagra diamond-shaped tablet shown in Pfizer's (now refused) Canadian trademark application 1,244,118 as follows:
One of the Federal Court's main concerns in that case was that the product as sold included additional features (such as the words "Viagra" and "Pfizer"):


The court felt that those additional features combined with shape and colour were necessary to act as an indicator of source. In other words, the combination of shape and colour alone were not sufficient to act as an indicator of source.
Similar challenges arise for pharmaceutical companies seeking to register or enforce trademark rights directed to the shape and colour of certain medical devices, such as the unusually-shaped two-tone, purple Advair inhaler, shown in Glaxo's (now expunged) Canadian trademark registration TMA687, 313 as follows:
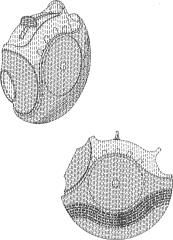
Similar to the Viagra case, one of the court's complaints was that, in spite of the unusual shape and colour combination, the Advair product as sold included additional features (such as the word "ADVAIR"):

The Federal Court held that in spite of massive sales, advertising and reputation, the evidence (in the court's view) did not establish that the shape/colour combination of the two-tone, purple Advair inhaler was perceived as an indicator of source.
While it may still be possible to register and enforce such marks, pharmaceutical companies need to understand that evidence that would be sufficient to establish distinctiveness in any other industry, may not be sufficient in these cases. More specifically, whereas evidence of a significant reputation is typically sufficient for any other industry, such evidence alone may not be sufficient in the case of the shape and colour of pharmaceuticals (or related products). To register and/or enforce such marks, it may also be necessary to establish trademark significance, namely that patients, doctors and pharmacists recognize the mark (and the mark alone) as an indicator of source.
Description of pharmaceuticals in trademark applications
The Canadian Trademarks Act requires that all goods in a trademark application be described in both "specific" and "ordinary commercial terms." While the Trademarks Office is not particularly strict about enforcing the "ordinary commercial terms" requirement, the Trademarks Office does strictly enforce the "specific" requirement. This means that for a trademark application listing a pharmaceutical, the Trademarks Office typically requires (a) a description of the specific conditions to be treated by the pharmaceutical or else (b) an indication of the specific type of medication. The requirement for detail in Canadian pharmaceutical trademark specifications is possibly the most strict of any trademark system worldwide.
Restrictions on advertising
An issue closely related to trademarks is that pharmaceutical companies face strict, sui generis, limitations under the Food and Drug Regulations upon how pharmaceuticals may be advertised in Canada.
Important changes under Canada's new Trademark Law
As noted above, Canadian trademark law will (or is at least expected to) change in 2019. While these changes will not uniquely affect the pharmaceutical industry, a number of changes will have important effects upon companies seeking to protect pharmaceutical marks in Canada.
Elimination of the need to file a declaration of use
Once the new law is in force, it will no longer be necessary to file a declaration of use. When the law changes, any pending applications being held up because of the need to file a declaration of use, will proceed directly to registration upon payment of the registration fee. In other words, under the new law, use will no longer be a requirement to obtain a trademark registration.
That having been said, it is important to understand that "use" will remain a fundamental attribute of Canadian trademark law. For example, once a trademark registration is at least three years old, anyone can initiate a section 45 (summary, non-use cancellation) proceeding, requiring the trademark owner to prove use (typically sales) in Canada within the preceding three years. If there is no evidence of use within the preceding three years, the registration will only be saved upon establishing "special circumstances" which are typically circumstances beyond the owner's control. For example, the need to await regulatory approval from Health Canada would likely be considered a special circumstance sufficient to justify maintaining a registration in spite of a lack of sales within the preceding three years.
New non-traditional marks
Currently, it is only possible to register a limited range of "non-traditional marks," such as shape, colour as applied to a particular shape and sound.
When the law changes in 2019, it will, at least in theory, be possible to register any and all non-traditional marks, such as scent, texture, colour per se, etc. However, it will be necessary to establish "distinctiveness" (essentially secondary meaning) as of the date of the trademark application.
Sound marks
One interesting change relating to non-traditional marks is worth specifically considering. Currently, it is possible to register sound marks without the need to establish distinctiveness. As noted above, once the law changes in 2019, it will be necessary to establish distinctiveness as of the Canadian filing date. Therefore, it is significantly easier to register sound marks now than it will be under the new law. For this reason, there is a tremendous advantage/benefit to seeking registration of sound marks now, before the new law comes into force.
For further information, please contact Philip Lapin or a member of our firm's Trademarks group.
The preceding is intended as a timely update on Canadian intellectual property and technology law. The content is informational only and does not constitute legal or professional advice. To obtain such advice, please communicate with our offices directly.
