The Indian Patents Act 1970 mandates requirement of unity of invention in the provisions of Section 10(5) of Act. As per the said provision the claim or claims of a complete specification should relate to a single invention, or to a group of inventions linked to form a single inventive concept. A single application may contain more than one independent claim, provided the claims are all of cognate character and are linked so as to form a single inventive concept.
Lack of Unity of Invention is observed when a single application includes multiple dependent claims directed at non-cognate aspects of the claimed invention. Also, if claims relates to plurality of distinct inventions, then such claims may be objected on ground of lack of unity of invention.
BIOTECHNOLOGICAL PATENT APPLICATIONS1
In case of gene technology inventions more often a large number of polynucleotide and polypeptide sequences are claimed in a patent application. Claiming a number of polynucleotide/polypeptide sequences often leads to confusion during publication or examination or searching stage as to whether claimed sequences relate to a single invention or to a group of inventions linked so as to form a single inventive concept.
LACK OF UNITY IN BIOTECHNOLOGICAL PATENT APPLICATIONS
There are two ways of determining of lack of unity in an application namely,
i. Before consideration of prior art, 'A priori' is established if the claims falling in different groups do not share a same or corresponding technical feature.
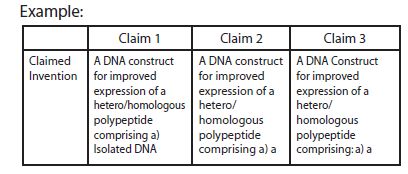
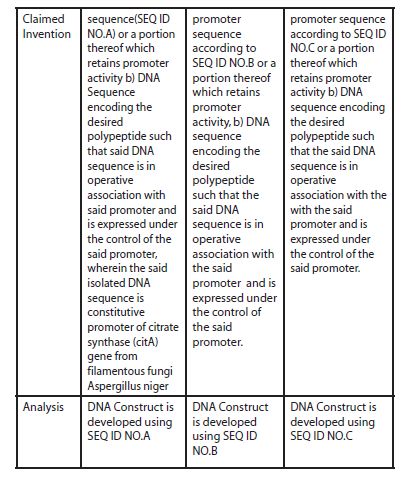
An analysis of the above claims shows that the DNA sequences as described as SEQ ID NO.A, B & C do not share any common structural feature. There is no technical feature that binds the aforesaid group. Therefore, each of the above groups is considered as separate inventions and hence lacks unity a priori.
ii. After the search of the prior art, 'A posteriori' is established, if the shared technical feature fails to make a contribution over the prior art.
Example:

An analysis of the claims in the above example shows that Claim 1 and 2 are two separate groups of inventions which are linked by a technical feature "X". When the technical feature "X" is subjected to prior art analysis, it is found that "X" is already known in the prior art. Therefore it is evident that this feature is not a special technical feature since it does not contribute to the technical advancement over the prior art. Since the claim 1 and 2 do not comply with Section 10(5) of the Act, the application lacks unity a posteriori.
PHARMACEUTICAL PATENT APPLICATIONS2
Pharmaceutical patent applications usually contains claim, that cover huge number of chemical compounds by Markush structures, chemical compounds as intermediate and final products, compositions comprising different chemical components, processes for their manufacture, their uses or applications, devices or apparatus used for carrying out processes are usually claimed in a single application. Markush claims impose an arduous task to handle search and examine such combination of different categories of claims.
DETERMINATION OF UNITY OF INVENTION IN MARKUSH CLAIMS
Unity of Invention criteria in case of Markush claims can be considered as satisfied only the alternatives that are claimed are of similar nature. The Markush group of alternative chemical compounds is subjected to the following conditions so as to decipher their similar nature.
I. Markush group of alternative compounds should share a common property or activity
II. All the alternatives should have a common structure, which is a significant structural element shared by all of the alternatives.
Example:
A compound of the formula R1-R2-R3 (useful for the treatment of asthma)
Where, R1 is an indolyl moiety
R2-R3 is methyl, benzyl or phenyl. Compound A has significant structural element that is shared by all of the alternatives and all the claimed alternatives possess the same activity. Hence a unity is established between all the alternatives of the Markush group of compounds.
DETERMINATION OF UNITY OF INVENTION IN INTERMEDIATE AND FINAL PRODUCT
"Intermediate" is a term that includes both intermediate and starting products which are used in a process to produce the final product through a physical or chemical change in which the identity of the intermediate is lost. The following criteria are applied in deciding the existence of unity of invention in intermediate and final product of a pharmaceutical invention,
- The intermediate and final product should have the same structural essential element, i.e the basic chemical structure of the intermediate and the final product should be the same, or the chemical structure of the intermediate and final product should be technically closely interrelated, with the intermediate incorporating an essential structural element to the final product.
- The term "technically interrelated", means that the final product is manufactured directly from the intermediate or is separated from it by a small number of intermediates all sharing the same essential structural element.
- To establish unity of invention between intermediate and final products when any one or both the structures are not known, sufficient evidence has to be provided to conclude that the intermediate and final products are technically closely interrelated such as the intermediate contains the same essential element as the final product or incorporates the essential element of the final product.
- Presence of same essential structural element in different intermediate products used in different processes for the preparation of the final product is a necessary criterion to satisfy unity of invention.
- Unity of Invention is identified if the intermediate and the final product are not separated in the process by an intermediate which is not new.
- Different intermediates for different structural parts of the final product do not satisfy unity of invention.
- Where the intermediate and the final products are families of compounds, each intermediate should correspond to a compound claimed in the family of the final products in order to satisfy unity of invention.
- Demonstration of other properties or activities in the intermediates should not affect the unity of invention.
CONCLUSION
A patent application cannot be denied protection due to lack of unity of invention, unlike in the case where the invention is found to be lacking in novelty and inventive step. A divisional application can be filed for the second and further inventions. On the other hand, the patent applicant can resort to technical argument to prove the existence of unity of invention in his/her application.
Footnotes
1. http://www.ipindia.nic.in/whats_new/biotech_Guidelines_25March2013.pdf
2. http://www.ipindia.nic.in/iponew/Guidelines_Pharma_PatentApplication_28February2014.pdf
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

